zinc stearate reaction
It is a white powder and is insoluble in water. At the same time it also has the function of vulcanization activator and softener in rubber.

Pdf Zinc Stearate Production By Precipitation And Fusion Processes Semantic Scholar
Excerpt from NIOSH Pocket Guide for Zinc stearate.
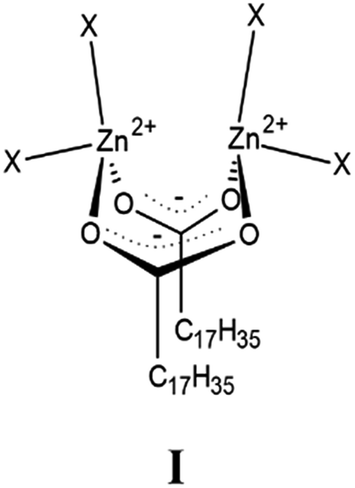
. Addition of zinc stearate is known to be technically a superior acid scavenger since it helps to avoid early discoloration of a large series polymer formulations but it holds for being physically irritant in humans. The reaction equation is. The technology comprises the following steps.
Caprate 050 Laurate 25 Palmitate 28-57 Stearate 28-68 Myristic 3-5 Pentadecanoic up to 050 Margaric Acid up to 2 Oleic acid up to 050 Arachidic and Behenic acid 30-40 Lauric Acid up to 050 Typical. The formation of mesh network is determined to occur slightly earlier than that of the network domains using a combination of in situ zinc K-edge XAFS and DSC measurements. Chmical reaction when stearic acid add KOH.
Adding zinc oxide four times and reacting at a temperature of 160DEG C under a pressure of 02Mpa for 50min. Safe handling of zinc stearate is discussed. In residues that had been held at 200 oC for 30 min prior to quenching distinct zinc stearate peaks were present.
Zinc stearate is produced from various reactions including the reaction of zinc oxide and stearic acid which is shown in the following. Adding molten zinc stearate into a tablet. 367 The solid residue quenched from 600 after reaction of ethyl stearate with zinc powder gave X-ray diffraction patterns for both zinc.
Electrocatalytically Active GraphenePorphyrin MOF Composite for Oxygen Reduction Reaction. Journal of the American Chemical Society. Stearate chemical properties physical properties and the application in rubber.
LOW Cancer LOW Allergies Immunotoxicity LOW Developmental and Reingredientive Toxicity LOW Use Restrictions Ingredient concerns CONCERNS DATA SOURCES Products with this Ingredient. Mainly used as lubricant and release agent for styrene resin phenolic resin and amino resin. Raising temperature from room temperature RT to 45C or 90C allowed the reaction to proceed faster.
When reacting stearic acid and zinc hydroxide hydrochloric acid is further added to the mixture as a catalyst. Zn stearate has a lower melt point 120C compared to Ca stearate mp 155 C. Zinc stearate has different ratios of palmitic and stearic acids.
Stearic acid reacts with potassium hydroxide to form water and potassium stearate. It is valued by manufacturers because of the delicate feeling it gives to the finished products good heat resistance excellent transparency yellowing resistance quick-drying and increased sandability. Zinc stearate can be used as a water-repellent a protective agent used in powders and ointments in the treatment of eczema acne and other skin diseases1.
Zinc oxide doesnt react with stearic acid. Zinc stearate is an organic substance with a chemical formula of C36H70O4Zn. A preparation technology of zinc stearate comprises the following steps that 1 glyceryl tristearate and water are hydrolyzed under the action of a catalyst and an antioxidant.
2 zinc oxide and a catalyst are added stepwise condensing dewatering and salt forming are conducted and zinc stearate is synthesized. Zinc stearate C36H70O4Zn is a compound with variable proportions of stearic and palmitic fatty acids. Method of producing zinc stearate involves reacting stearic acid and zinc hydroxide with heating and intense stirring followed by heat treatment filtration drying and packaging.
Conveying liquid stearic acid by a pump allowing the liquid stearic acid to go through a flow meter for metering and then enter a zinc stearate reaction vessel stirring and heating. Zinc stearate is high in preparation yield good in color and cluster low. 12 This is probably due to the acceleration of sulfur cross-linking of the mesh network by the dinuclear bridging bidentate zincstearate complex intermediate.
Zinc Stearate is a material that is easily soluble in water ultra-fine and with good dispersion compatibility. 1214 In the previous section ArH. Introduction Fatty acid metal salts or soaps play an important role as a process aid.
This chemical is an example of metal soaps and is insoluble in water and polar solvents such as alcohol. These stearates are viewed as salts or soaps in general terms. Considering the molar masses of each chemical substance in the reaction gmol -1 Zn 814 gmol -1 stearic acid 2845 gmol -1 and water 18 gmol -1 and the respective stoichiometric.
Inhalation ingestion skin andor eye contact. Toluene helped zinc stearate to turn into the corresponding methyl esters by making zinc stearate partly soluble in a raised temperature as well as making the reaction product soluble. Because zinc oxide is insoluble in rubber its active effect cannot be fully exerted when used alone.
It needs to be used in combination with stearic acid to produce rubber-soluble zinc soap zinc stearate and then participate in the vulcanization reactionStearic acid has an activating effect which has an acidic activating effect on the double bonds of rubber. Eyes skin respiratory system NIOSH 2022. For the purpose of this paper we will refer to them as salts.
In samples formed from zinc oxide and the ester zinc stearate was detected similarly after reaction at 200 oC for 30 min. 54 Exposure to zinc stearate over a long time may develop extensive fibrosis. These salts are produced from a reaction with stearic.
Common concerns See how this product scores for common concerns. The aqueous emulsion of zinc stearate is called zinc stearate. Certain blends of Ca and Zn stearate have even lower melt point ranges between 100.
Zinc Stearate is a zinc salt of stearic acid a naturally occurring fatty acid. Irritation eyes skin upper respiratory system. Although there is no specific information available on the concentration of the exposure.
Zinc stearate is made by reacting sodium stearate with a solution of zinc sulfate.

Novel Approach For Rapid Oil Water Separation Through Superhydrophobic Superoleophilic Zinc Stearate Coated Polyurethane Sponges Sciencedirect

Influence Of Two Different Alcohols In The Esterification Of Fatty Acids Over Layered Zinc Stearate Palmitate Sciencedirect

Scielo Brasil Influence Of Zno On The Properties Of Elastomeric Compositions And Their Leached Extract Influence Of Zno On The Properties Of Elastomeric Compositions And Their Leached Extract

Catalysts Free Full Text Zinc Based Curing Activators New Trends For Reducing Zinc Content In Rubber Vulcanization Process Html
Zinc Stearate C36h70o4zn Pubchem
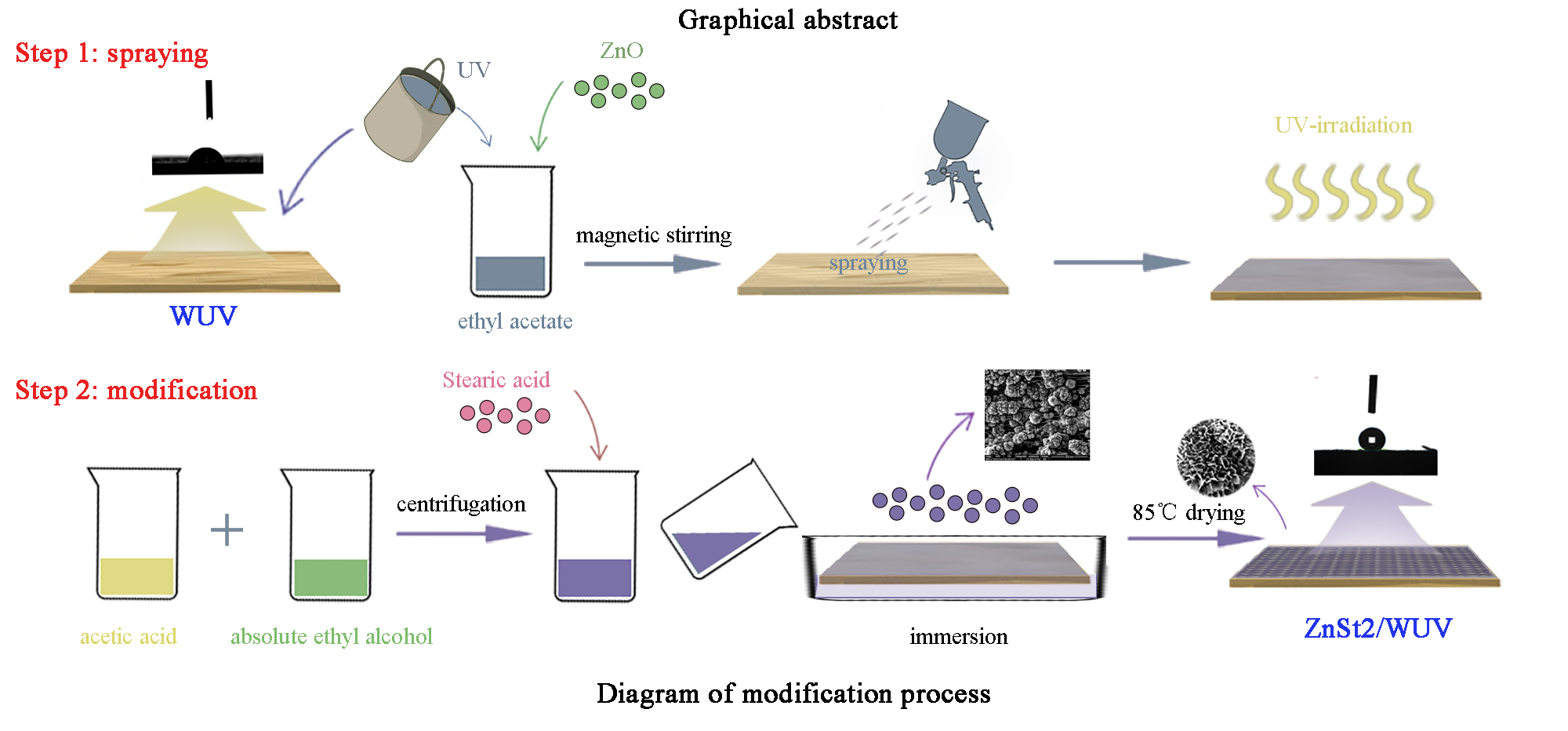
Polymers Free Full Text Preparation And Characterization Of Waterborne Uv Lacquer Product Modified By Zinc Oxide With Flower Shape Html

Effect Of Synthesized Zinc Stearate On The Properties Of Natural Rubber Vulcanizates In The Absence And Presence Of Some Fillers Sciencedirect

Measured Characteristics Of The Prepared Zinc Stearate Download Table

Zinc Stearate Purum Zn 10 12 557 05 1

Reaction Between Zno And Stearic Acid Forming Zinc Stearate And The Download Scientific Diagram

Tga Dsc Curves Of Zinc Stearate Reveal A Relative Thermal Equilibrium Download Scientific Diagram

Reaction Between Zno And Stearic Acid Forming Zinc Stearate And The Download Scientific Diagram

Effect Of Fatty Acids On The Accelerated Sulfur Vulcanization Of Rubber By Active Zinc Carboxylate Complexes Rsc Advances Rsc Publishing Doi 10 1039 C9ra10358a
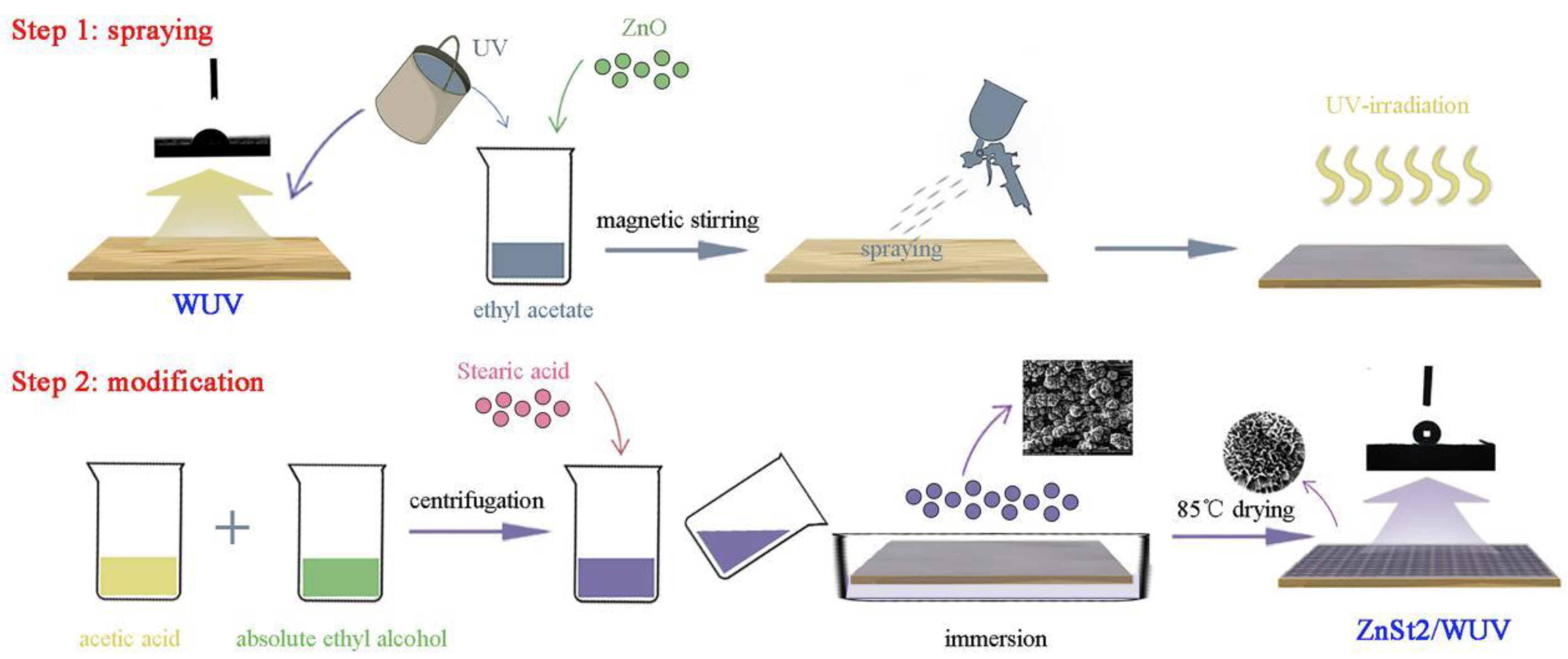
Polymers Free Full Text Preparation And Characterization Of Waterborne Uv Lacquer Product Modified By Zinc Oxide With Flower Shape Html
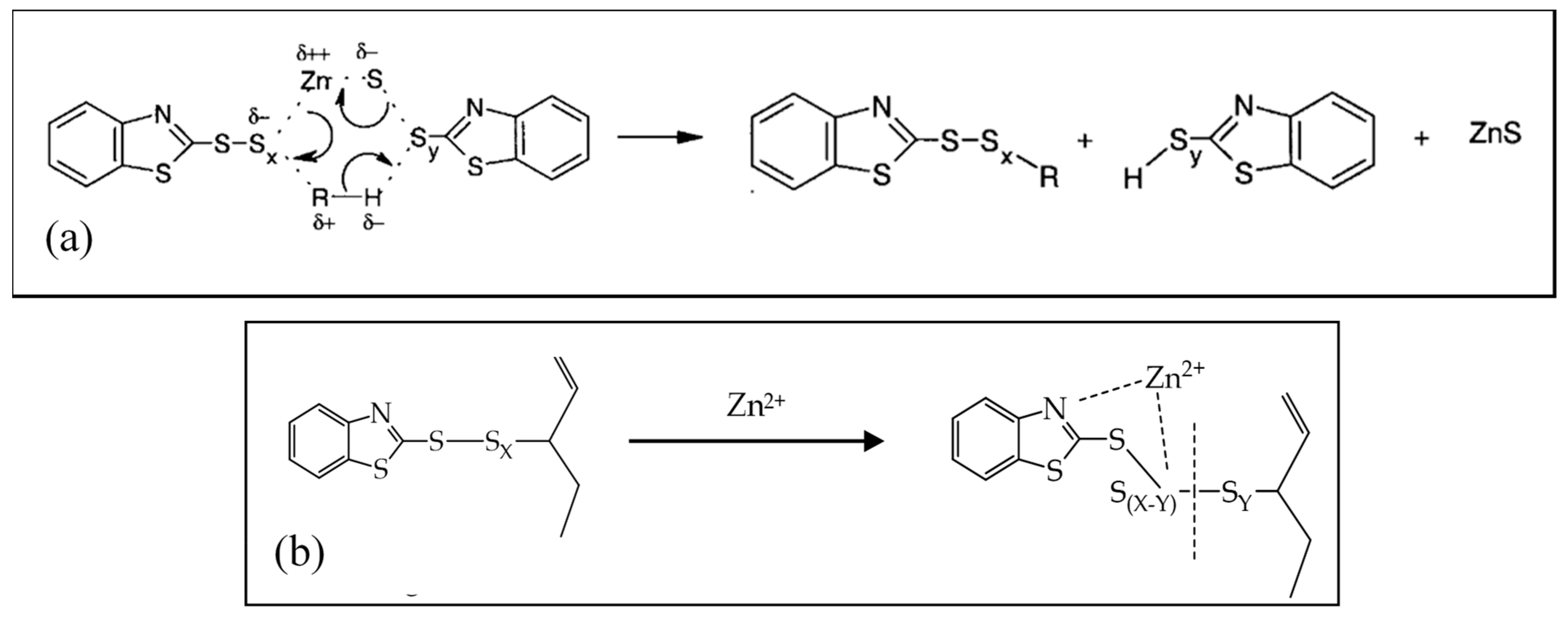
Catalysts Free Full Text Zinc Based Curing Activators New Trends For Reducing Zinc Content In Rubber Vulcanization Process Html

Zinc Oxide Surface A Versatile Nanoplatform For Solvent Free Synthesis Of Diverse Isatin Derivatives Sciencedirect
Reaction Between Zno And Stearic Acid Forming Zinc Stearate And The Download Scientific Diagram

Zinc Stearate Formation And Vulcanization Mechanisms For Step 1 Download Scientific Diagram
Comments
Post a Comment